A Mixture is Easy to Separate Parts of Mixture Include Filtration
Learning Objectives
- Describe techniques for separation of mixtures.
How did goldminers search for gold?

How did goldminers search for gold?
Beginning in the late 1840s, thousands of prospectors rushed to California to search for gold. One of the approaches taken to isolate the gold from the soil was called "panning." Dirt would be placed in the pan and covered with water. After thorough mixing, the pan is gently swirled to remove dissolved material while the heavier gold settles to the bottom of the pan. The gold is then separated from the mixture of soil and water.
Separation of Mixtures
Not everyone is out searching for gold (and not many of those searchers is going to get much gold, either). In a chemical reaction, it is important to isolate the component(s) of interest from all the other materials so they can be further characterized. Studies of biochemical systems, environmental analysis, pharmaceutical research – these and many other areas of research require reliable separation methods.
Here are a number of common separation techniques:
Chromatography
Chromatography is the separation of a mixture by passing it in solution or suspension or as a vapor (as in gas chromatography) through a medium in which the components move at different rates. Thin-layer chromatography is a special type of chromatography used for separating and identifying mixtures that are or can be colored, especially pigments.
Distillation
Distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation is a purification process where the components of a liquid mixture are vaporized and then condensed and isolated. In simple distillation, a mixture is heated and the most volatile component vaporizes at the lowest temperature. The vapor passes through a cooled tube (a condenser), where it condenses back into its liquid state. The condensate that is collected is called distillate.
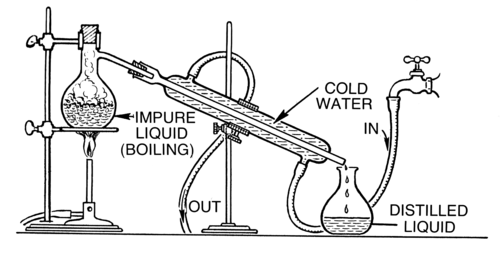
Figure 2.11
Distillation apparatus.
In the Figure above , we see several important pieces of equipment. There is a heat source, a test tube with a one-hole stopper attached to a glass elbow and rubber tubing. The rubber tubing is placed into a collection tube which is submerged in cold water. There are other more complicated assemblies for distillation that can also be used, especially to separate mixtures, which are comprised of pure liquids with boiling points that are close to one another.
Evaporation
Evaporation is a technique used to separate out homogenous mixtures where there is one or more dissolved solids. This method drives off the liquid components from the solid components. The process typically involves heating the mixture until no more liquid remains, Prior to using this method, the mixture should only contain one liquid component, unless it is not important to isolate the liquid components. This is because all liquid components will evaporate over time. This method is suitable to separate a soluble solid from a liquid.
In many parts of the world, table salt is obtained from the evaporation of sea water. The heat for the process comes from the sun.

Figure 2.12
Once the sea water in these evaporation ponds has evaporated, the salt can be harvested.
Filtration
Filtration is a separation method used to separate out pure substances in mixtures comprised of particles some of which are large enough in size to be captured with a porous material. Particle size can vary considerably, given the type of mixture. For instance, stream water is a mixture that contains naturally occurring biological organisms like bacteria, viruses, and protozoans. Some water filters can filter out bacteria, the length of which is on the order of 1 micron. Other mixtures, like soil, have relatively large particle sizes, which can be filtered through something like a coffee filter.
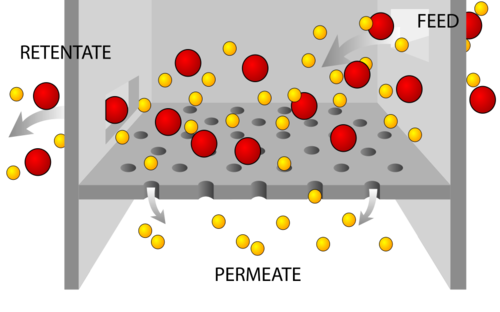
Figure 2.13
Filtration.
Summary
- Mixtures can be separated using a variety of techniques.
- Chromatography involves solvent separation on a solid medium.
- Distillation takes advantage of differences in boiling points.
- Evaporation removes a liquid from a solution to leave a solid material.
- Filtration separates solids of different sizes.
Practice
Questions
Use this resource to answer the following questions:
http://www.gourmetsleuth.com/Articles/Exotic-Herbs-Spices-and-Salts-639/hawaiian-salt.aspx
- Where is salt produced in the Hawaiian Islands?
- What does "paakai" mean?
- How long does it take for salt to form by evaporation?
Review
Questions
- Why is it important to separate material from a mixture?
- What is chromatography?
- What is distillation
- What is filtration?
- What is evaporation?
- What technique would you use to separate sand from water? There are two possibilities.
- What technique would you use to separate alcohol from water?
Glossary
- chromatography: Involves solvent separation on a solid medium.
- distillation: Takes advantage of differences in boiling points.
- evaporation: Removes a liquid from a solution to leave a solid material.
- filtration: Separates solids of different sizes.
Show References
Source: https://www.coursehero.com/study-guides/cheminter/methods-for-separating-mixtures/
0 Response to "A Mixture is Easy to Separate Parts of Mixture Include Filtration"
Post a Comment